
Background
The criteria of molecular relapse in different treatment-free remission (TFR) trials in patients (pts) with chronic myeloid leukemia (CML) varied. In the early trials molecular relapse was defined as loss of deep molecular response (DMR) including MR4 loss. In recent ELN guideline (Hochhaus et al, 2020) major molecular response (MMR) loss was a criterion of molecular relapse. We consider it reasonable to evaluate the role of MR4 loss in connection with MMR loss and time of treatment-free observation and to describe the pattern of minimal residual disease (MRD) in this context.
Aim
To evaluate the role of MR4 loss on further MMR loss in CML pts during early and late period after TKI cessation and to describe the pattern of MRD during TFR.
Patients and methods
In total 98 CML pts with chronic phase who had received therapy by any TKI ≥3 years (yrs) with sustained DMR (at least MR4 or BCR-ABL ≤0.01% by international scale (IS)) during ≥2 yrs) were enrolled into the prospective TFR study RU-SKI. The BCR-ABL level (IS) was evaluated by RQ-PCR monthly during the first 6 months (mo) after TKI cessation, every 2 mo from 6 to 12 mo and every 3 mo thereafter. MR4 loss was considered if BCR-ABL was >0,01% and <0,1%. TKI were resumed in pts with molecular relapse which was considered as MMR loss (BCR-ABL>0,1%). Molecular relapse free survival (MRFS) was evaluated by Kaplan-Meier method, log-rank test was used for comparison.
Results
Меdian (Me) time after TKI discontinuation was 40 mo (range 28-57). MMR loss and MR4 loss was observed in 48(49%) and 38(39%) pts respectively. In 42(87%) pts MMR loss was observed within first 6 mo after treatment cessation. MRFS at 36 mo was 51% (95% CI 41 - 61%).
No MMR loss occurred in 10(26%) pts with MR4 loss while 28(74%) pts with MR4 loss subsequently lost MMR. The MRFS after the first documented MR4 loss was 29% (95% CI 15 - 44%) and 24% (95% CI 10,5 - 38%) at 12 and 24 mo respectively.
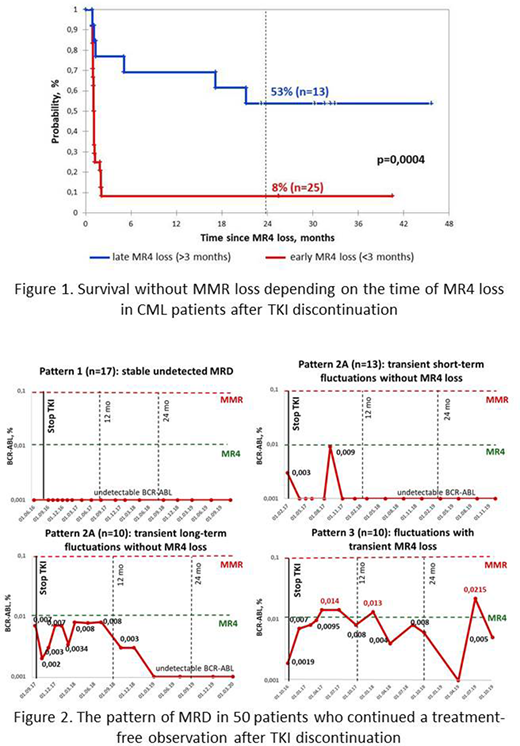
MRFS at 24 mo was significantly higher in pts with late MR4 loss (>3 mo) than in pts with early MR4 loss (<3 mo): 53% and 8% respectively. (р=0,0004, figure 1).
The pattern of MRD in 50 pts who continued a treatment-free observation after TKI cessation was represented by 3 main variants: 1) stable undetected MRD in 17(34%) pts; 2) transient short-term or long-term fluctuations without MR4 loss in 23(46%) pts; 3) fluctuations with transient MR4 loss but without MMR loss in 10(20%) pts (figure 2). Interestingly, 8 of 10 pts with MR4 fluctuations subsequently achieved MRD-negative status.
Conclusion
We confirmed that MR4 loss after TKI cessation in most cases (74%) preceded the molecular relapse which was considered as MMR loss. However, the MRFS was significantly higher in pts with late MR4 loss than in those with early MR4 loss during the first 3 mo of TKI cessation and comparable with MRFS in the whole pts cohort.
We found that only 34% pts who maintained a molecular remission had stable undetected MRD while 66% of pts had fluctuations of MRD during TFR. About a quarter of pts (26%) with MR4 loss could remain in TFR and achieve the MRD-negative status. We support the importance of regular MRD monitoring both in early and late terms during TFR observation to benefit for the safety of the pts.
Chelysheva:Novartis: Other: performed lectures; Fusion Pharma: Consultancy. Shukhov:Novartis: Consultancy, Other: performed lectures; Pfizer: Consultancy, Other: performed lectures. Ionova:BMS: Other: principal investigator of the observational studies sponsored by BMS; Takeda: Other: principal investigator of the observational studies sponsored by Takeda. Turkina:Pfizer: Honoraria; Novartis Pharma: Honoraria; BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal